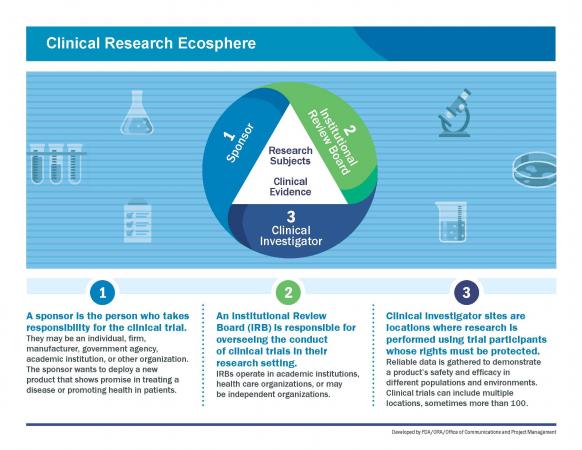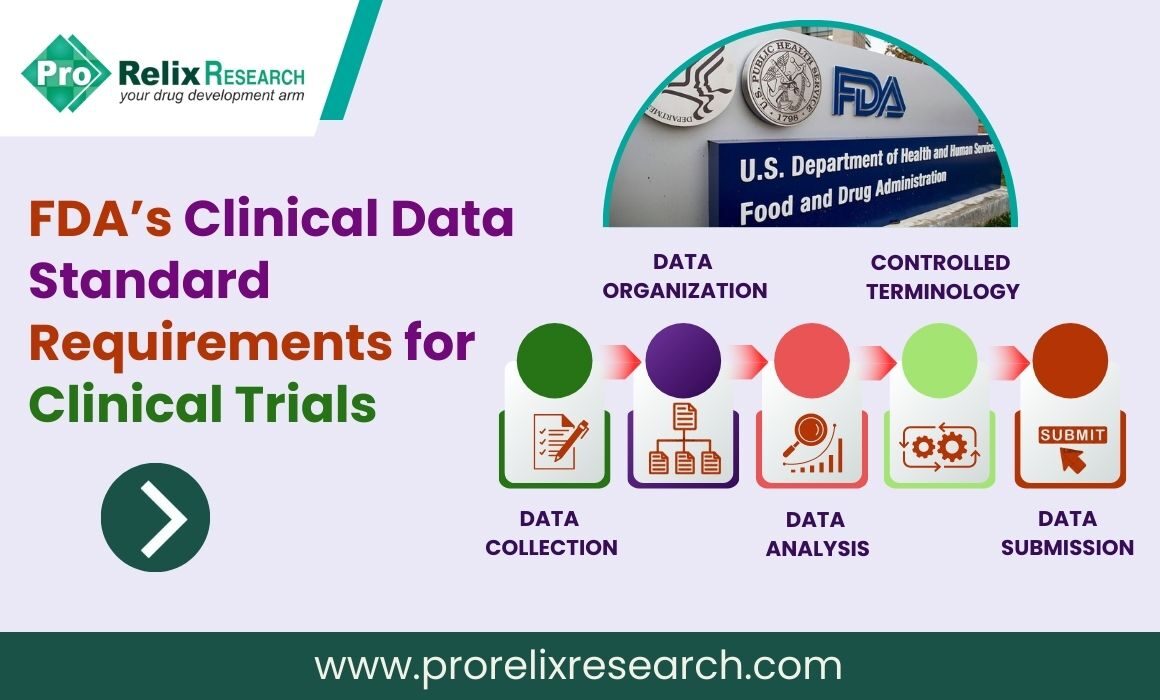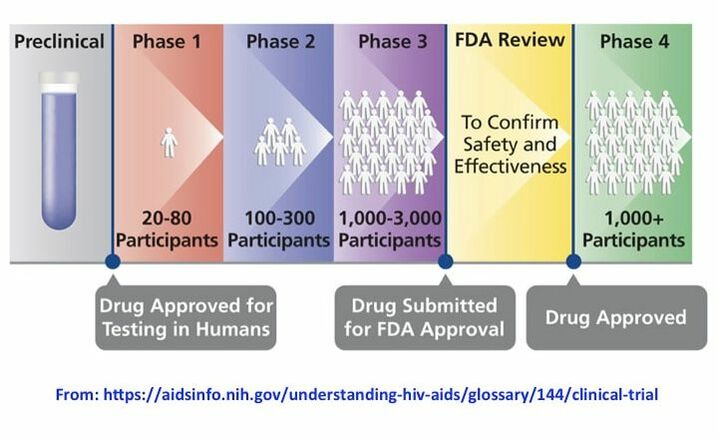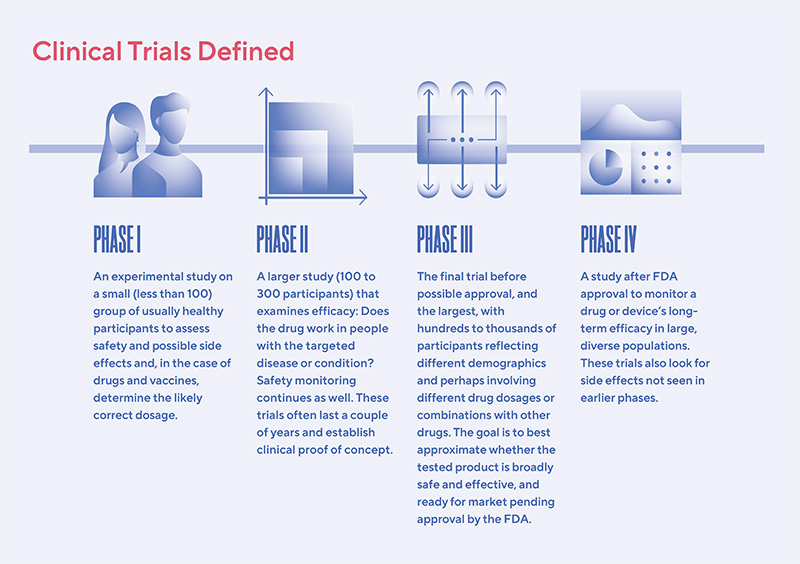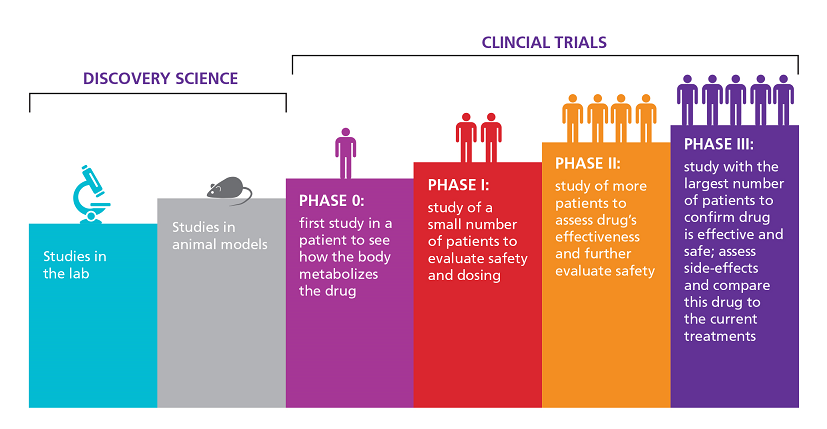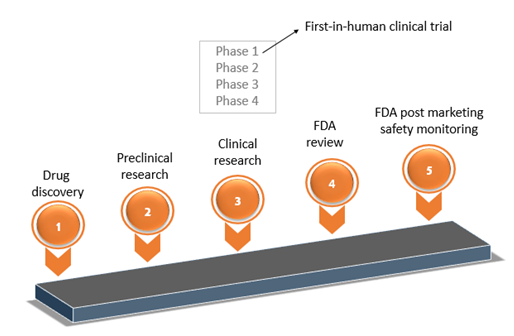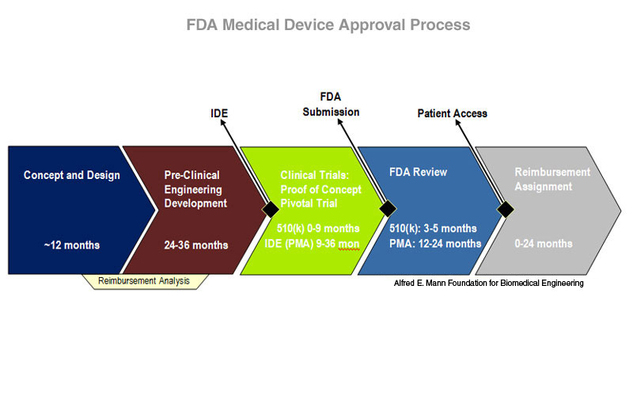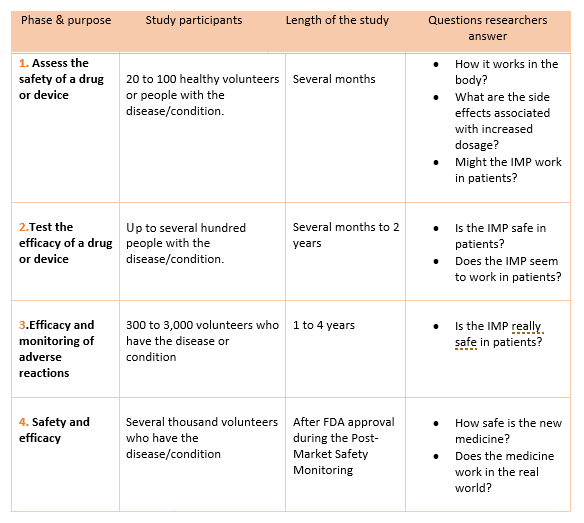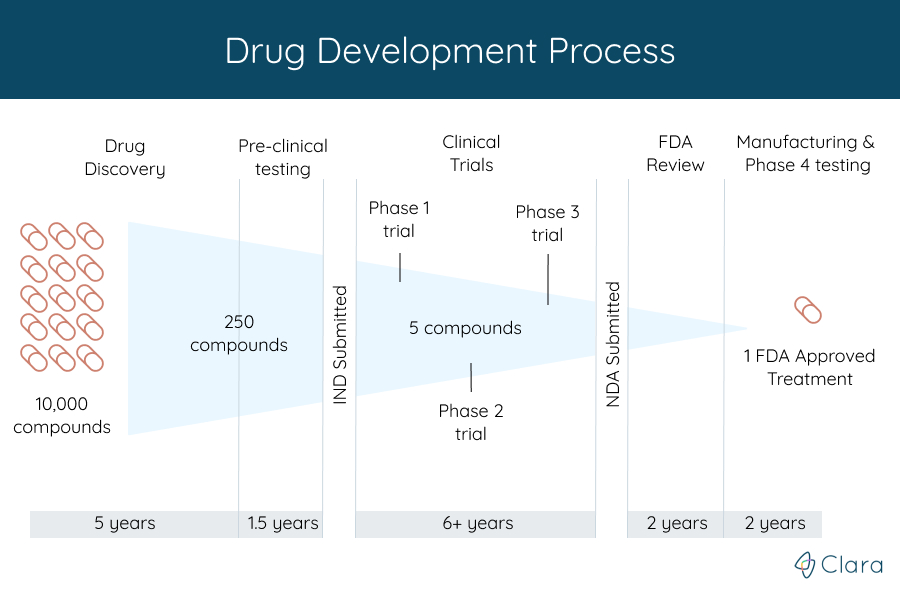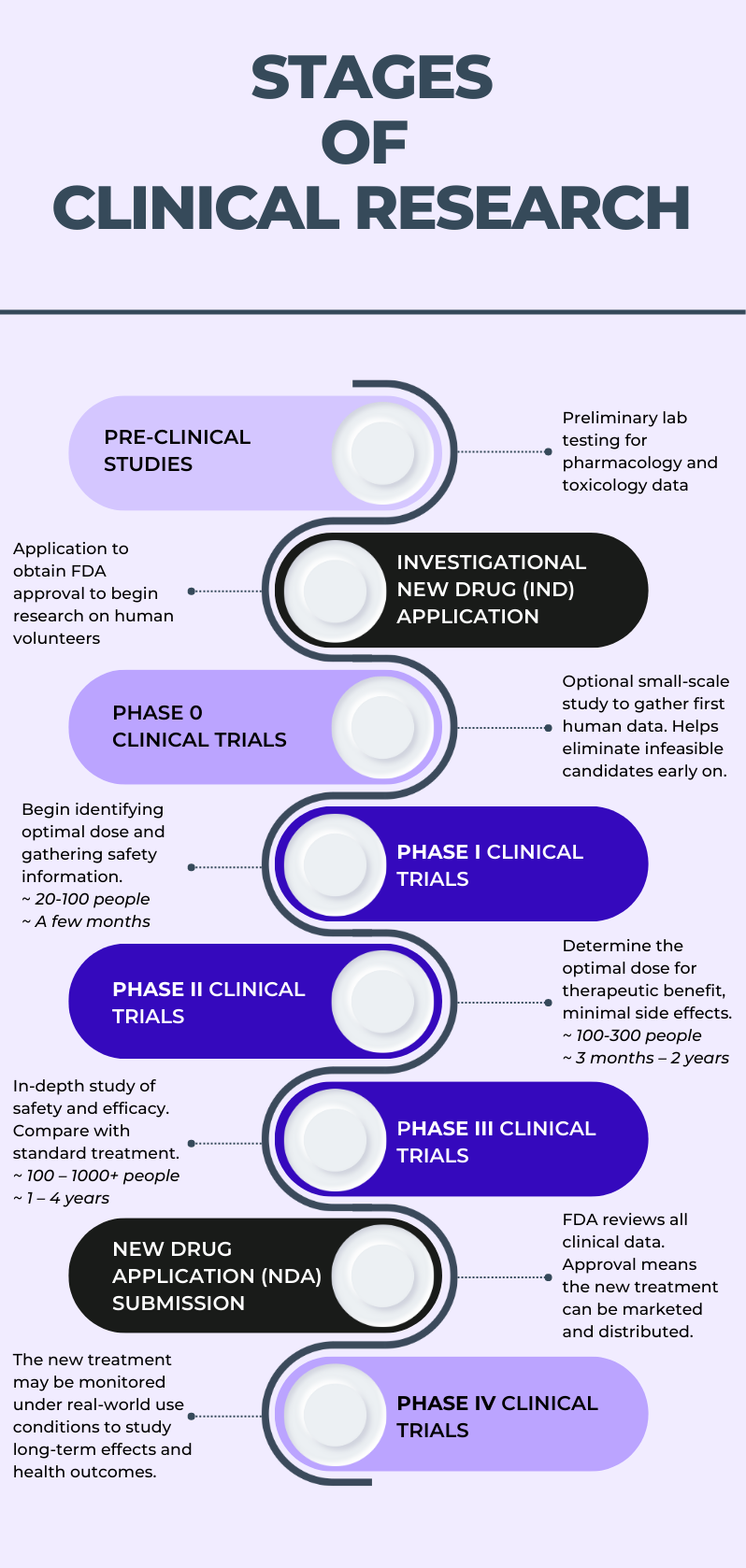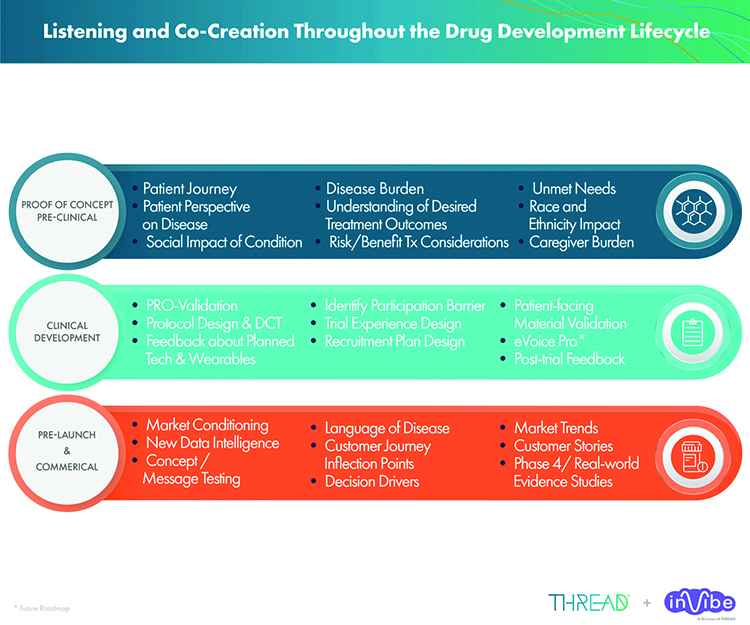
The FDA weighs in on market research and drug developers are listening – especially to the underserved - PharmaLive

An overview of the procedure for clinical trial applications and the... | Download Scientific Diagram

Sample construction for pivotal trials using surrogate markers with... | Download Scientific Diagram