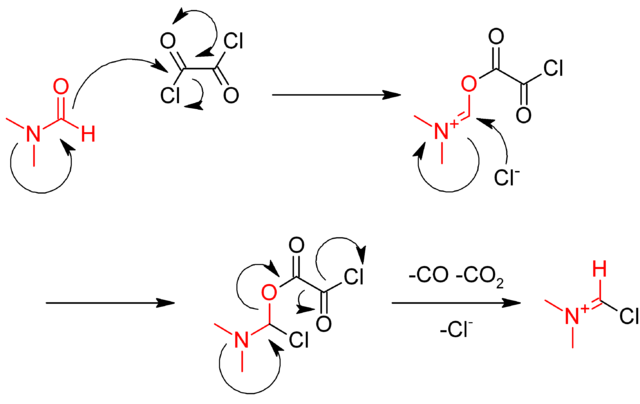
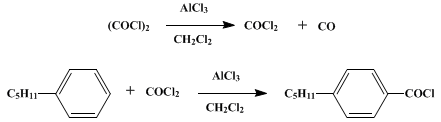
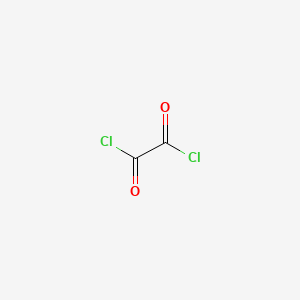
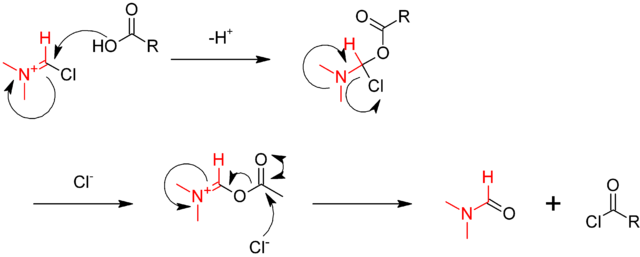
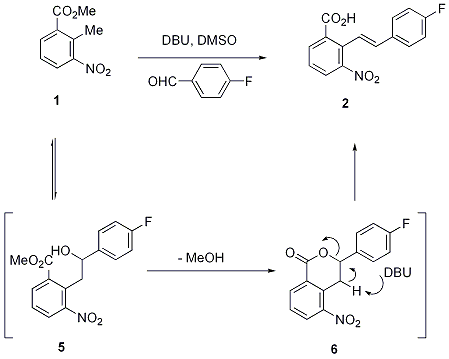
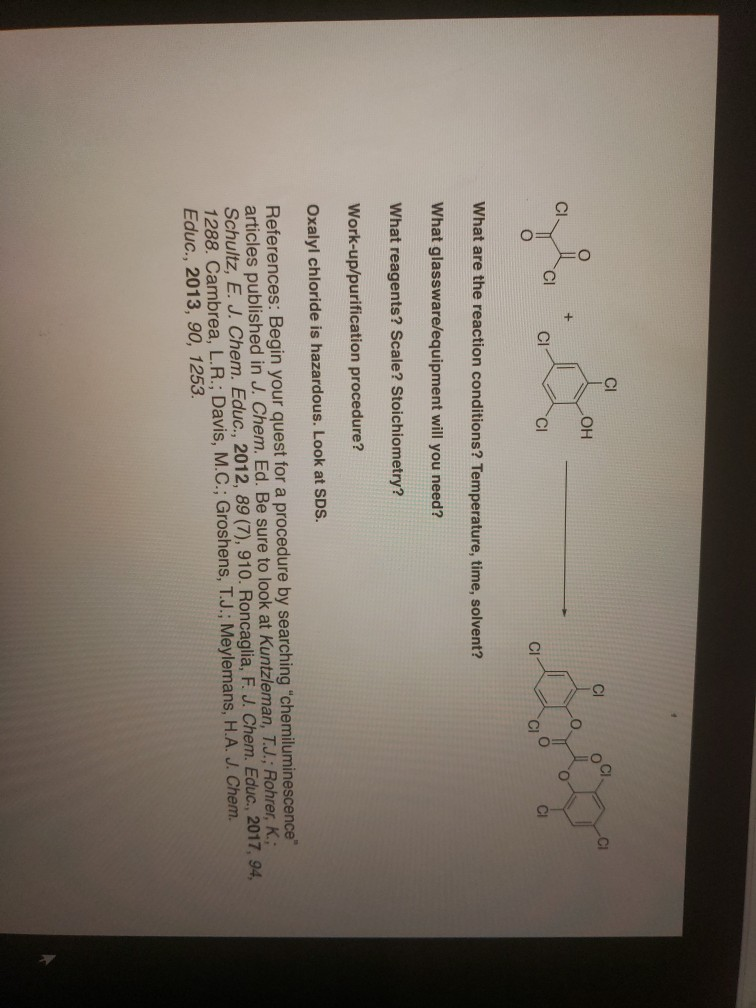
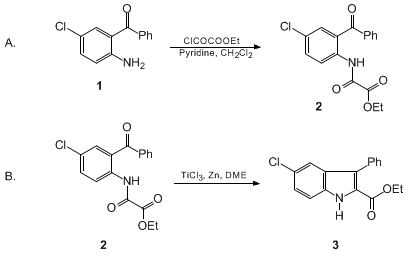
Oxalyl Chloride: A Versatile Reagent in Organic Transformations - Mohammadkhani - 2019 - ChemistrySelect - Wiley Online Library

Oxalyl Chloride: A Versatile Reagent in Organic Transformations - Mohammadkhani - 2019 - ChemistrySelect - Wiley Online Library

A novel and highly efficient esterification process using triphenylphosphine oxide with oxalyl chloride | Royal Society Open Science

Alcohols as Latent Coupling Fragments for Metallaphotoredox Catalysis: sp3–sp2 Cross-Coupling of Oxalates with Aryl Halides | Journal of the American Chemical Society

The Conversion of tert-Butyl Esters to Acid Chlorides Using Thionyl Chloride | The Journal of Organic Chemistry

Scheme 1. Reagents and conditions: (a) oxalyl chloride, chlorobenzene,... | Download Scientific Diagram

Stereoretentive cross-coupling of chiral amino acid chlorides and hydrocarbons through mechanistically controlled Ni/Ir photoredox catalysis | Nature Communications

Molecules | Free Full-Text | Synthesis of Anti-Inflammatory Spirostene-Pyrazole Conjugates by a Consecutive Multicomponent Reaction of Diosgenin with Oxalyl Chloride, Arylalkynes and Hydrazines or Hydrazones

Convenient synthesis and in vitro activity of oxalyl bis(benzenesulfonylhydrazides) and related compounds - ScienceDirect

A convenient and mild chromatography-free method for the purification of the products of Wittig and Appel reactions - Organic & Biomolecular Chemistry (RSC Publishing)

Oxalyl Chloride: A Versatile Reagent in Organic Transformations - Mohammadkhani - 2019 - ChemistrySelect - Wiley Online Library